My Chemistry Journal Part 4 (FINAL)









CONCENTRATION OF SOLUTIONS
Molarity
Molarity tells us the number of moles of solute in exactly one liter of a solution. (Note that molarity is spelled with an "r" and is represented by a capital M.)
We need two pieces of information to calculate the molarity of a solute in a solution:
- The moles of solute present in the solution.
- The volume of solution (in liters) containing the solute.
To calculate molarity we use the equation:
Molality
Molality, m, tells us the number of moles of solute dissolved in exactly one kilogram of solvent. (Note that molality is spelled with two "l"'s and represented by a lower case m.)
We need two pieces of information to calculate the molality of a solute in a solution:
- The moles of solute present in the solution.
- The mass of solvent (in kilograms) in the solution.
To calculate molality we use the equation:

Mole Fraction
The mole fraction, X, of a component in a solution is the ratio of the number of moles of that component to the total number of moles of all components in the solution.
To calculate mole fraction, we need to know:
- The number of moles of each component present in the solution.
The mole fraction of A, XA, in a solution consisting of A, B, C, ... is calculated using the equation:

To calculate the mole fraction of B, XB, use:



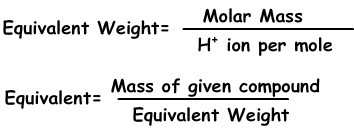
submitted by: Paulaine Cordon
SOF 3-Pearl
Last and Final Chapter of my chemstry journal..
related links:
http://www.asianfanfics.com/blog/view/271757
http://www.asianfanfics.com/blog/view/333745
http://www.asianfanfics.com/blog/view/423288
THANK YOU!!!!!!!!!!!!
Comments