My Chemistry Journal Part 2
The electron configuration is the distribution of electrons of an atom or molecule
Different Rules
1) Aufbau Rule
2) Orbital Notation
3) Energy Shell Notation

4) Half Shell Notation
5) Noble Gas Core Notation

6) Periodic Table Notation

Quantum Number- Define the energy state of an electron.
Principal quantum number: n
Allowed values: 1, 2, 3 …………………. ∞
Defines the size and approximate energy of the orbital.
Subsidiary quantum number: l
Allowed values: l = 0, 1, 2, 3 ………………. (n-1)
Defines the shape of the orbital and the fine details of the energy.
Magnetic quantum number: ml
Allowed values: -l ………… 0 ………… +l
Defines the allowed number of orbitals of a particular size (determined by n), a particular shape (determined by l) and a particular energy (determined by n and ltogether) which differ principally in their orientation in space.
Spin quantum number: s
Allowed values: ±1⁄2
PeriodicTrends

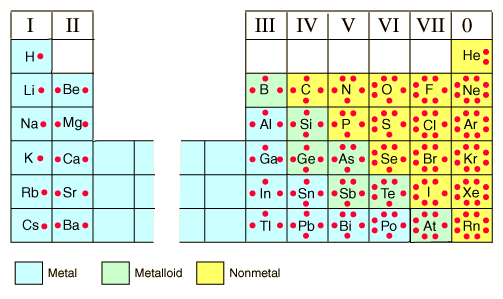
Lewis Dot Structures- show the symbol of the element surrounded byt the valence electrons represented by dots.
Covalent Bonds- two atoms sharing on one electron each.
2 types of Covalent Bonds
Polar Covalent

Non-polar Covalent

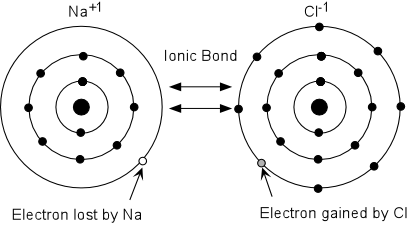
Ionic Bond
Molecular Geometry
Linear

Bent

Trigonal Planar

Tetrehedral

Pyramidal

Polyatomic Ions- species (ion) composed of two or more atoms covalently bonded

Naming Covalent Compounds

Naming Ionic Compounds


Naming Bases (ends in -OH)

Naming Acids (ends in H-)
Non-oxy Acid Hydro+non+ic acid
Oxy Acid H+polyatomic ion+ ite/ate+ acid
Chemical Formula
Criss Cross Method

Solving for oxidation number

Percentage Composition

submitted to:
Ms. Leonora Tingatinga
hope many of you can learn from this!!! :)

Comments